Agricultural Literacy Curriculum Matrix
Lesson Plan
Concentrate on the Solution
Grade Level
Purpose
In this lesson, students will use their knowledge of solutes, solvents, and parts per million to analyze fertilizer options that meet plant nutrient requirements while evaluating costs associated with managing plant nutrients. Grades 9-12
Estimated Time
Materials Needed
For each student:
- Concentrate on the Solution worksheet (Key)
- Periodic Table of the Elements
- Optional for Reference: Answers to Commonly Asked Questions
Vocabulary
amendment: any material added to soil to make it more productive such as fertilizer or compost
chemical element: a substance that cannot be broken down into any simpler chemical substances and is made of atoms all of the same type
concentration: the ratio of the mass or volume of a substance (solute) to the mass or volume of the solvent or solution
macronutrient: a nutrient that must be present in a relatively large amount to ensure the health of the organism; building blocks used to make essential biomolecules
micronutrient: a nutrient required in small quantities to ensure the health of the organism; often used as cofactors for enzymatic reactions
nutrient: a substance that provides nourishment essential for growth and the maintenance of life
periodic table: a chart in which the chemical elements are arranged based on atomic weights and chemical characteristics according to their atomic numbers
pH: power of hydrogen; a measure of the alkalinity or acidity of a substance
sustainable agriculture: an approach to agriculture that focuses on producing food while improving the economic viability of farms, protecting natural resources, and enhancing quality of life for farmers and society as a whole
Background Agricultural Connections
This lesson is one in a series of related lessons to introduce students to chemistry and environmental science concepts. Activities are modeled after real-life challenges that modern farmers face while producing our food, fiber, and fuel. Labs are inquiry based and promote critical thinking skills. Other related lessons include:
- One in a Million
- Concentrate on the Solution
- Matter of Fact
- What's Your pH?
- Know Your Nitrogen
The correct amount of plant nutrients is essential for growing healthy crops. How do farmers know if a crop should be fertilized and what type of fertilizer to use?
Collecting and testing multiple soil samples is a key step in determining whether fertilizer is needed to supplement nutrient deficiencies in the soil. Farmers compare soil test results to the nutrient requirements of their crops and then decide which fertilizer, if any, should be applied. If a fertilizer is needed, farmers make precise plans for choosing the right fertilizer, amount, application method, and timing. One type of fertilizer doesn’t fit all needs. Too much of one nutrient can cause deficiencies in other nutrients. For example, applying too much magnesium can interfere with plant potassium uptake. Also, excess application of fertilizer costs the farmer money and can lead to environmental problems. For these reasons, fertilizer production, transportation, application, and storage are heavily regulated by numerous agencies in California and throughout the U.S.
The continued development of farming technology and precise management of fertilizer inputs is necessary for farmers to grow more food on less land to feed our growing population. In 1910, the discovery of the Haber-Bosch process for fixing atmospheric nitrogen gas into commercial fertilizer drastically increased the amount of food that farmers could grow. It is estimated that nearly half the world's population is dependent upon commercially produced fertilizers.
There are 17 chemical elements that are essential to plants. Of these 17, carbon, hydrogen, and oxygen are provided to plants by air and water. Carbon is the basic building block for life and is taken in by plants in the form of carbon dioxide from the atmosphere. Through photosynthesis, carbon is combined with hydrogen and oxygen to form carbohydrates. The remaining 14 elements are absorbed by plant roots from the soil and are categorized as follows:
Primary Nutrients: Nitrogen, Potassium, and Phosphorus
- These are the three most commonly deficient nutrients in the soil. After carbon, hydrogen, and oxygen, plants take up more potassium and nitrogen than any other nutrient.
- Nitrogen is used by plants to make proteins, chlorophyll, and enzymes.
- Potassium is important for efficient water use by plants because it is required to open and close stomata. Potassium is also important for root growth and formation of starch.
- Phosphorus is needed for plants to make DNA and RNA.
- Secondary Nutrients: Calcium, Magnesium, and Sulfur
- Similar amounts of these nutrients are needed as the primary nutrients but are classified as secondary nutrients since the soil is less likely to be deficient in these nutrients.
- Calcium is important in the formation of new cell walls and membranes.
- Magnesium is essential in forming chlorophyll for photosynthesis.
- Sulfur is involved in protein synthesis and the formation of nitrogen fixing nodules on legume roots.
- Micronutrients: Zinc, Iron, Manganese, Copper, Boron,
- Molybdenum, Chlorine, and Nickel
- Micronutrients are just as important to plants as the primary and secondary nutrients, but are used in much smaller amounts.
Engage
- Ask the class if they have ever tried growing their own flowers, vegetables, or house plants. Ask how they kept their plants healthy. What did they do if their plants began to die or show signs of disease?
- Ask students if plants need nutrients just like people do. (Yes)
- Ask students how plants obtain nutrients. Allow students time to answer the question, then explain that they will:
- explore best practices associated with fertilizer use; and
- learn about plant nutrient requirements.
Explore and Explain
- Explain that farmers must know as much as possible about their land, especially their soil properties. Farmers collect soil samples and send them to laboratories for analysis. Soil properties are then compared with the needs of the crops the farmer plans to grow. If soil is lacking in one or more essential nutrients, fertilizer may be applied.
- Not all fertilizers contain the same amounts of primary nutrients. Fertilizers are prepared in different compositions so the consumer, farmer, or home gardener can apply precisely what is needed.
- In this lesson, students will take on the role of manager of their school farm. This job will require students to use their chemistry, math, and communication skills to compare the cost and nutrient content of different types of fertilizers in order to make the best recommendations for purchase of different fertilizers.
- Explain that soil sample results from the school farm indicate areas with different nutrient deficiencies. Students will need to determine which fertilizer to use in order to remedy these deficiencies. Their success will positively impact both crop yield and environmental health.
- Lead the class through a review of the periodic table of the elements as well as the characteristics of a compound:
- Elements can be combined to form a compound. An example of an element is hydrogen. An example of a compound is water (HO).
- A compound formula uses subscripts to indicate the number of atoms of each element present. For example, in the compound HO, there are two atoms of hydrogen and one atom of oxygen present.
- Compounds contain the same elements in the same proportions regardless of sample size.
- Explain that plant nutrients are typically added to the soil or growing media in compound forms. This means that instead of adding pure nitrogen (which would be difficult, since nitrogen in its pure form is a gas), we might add ammonium nitrate (NHNO), which is a solid, plant available form.
- Distribute the Concentrate on the Solution worksheet to each student and organize the class into groups of two or three. Assign groups one of the following nutrient deficiencies and the supplemental nutrient amount needed. For example, one group may be assigned phosphorus and the supplemental nutrient amount needed is 40 ppm.
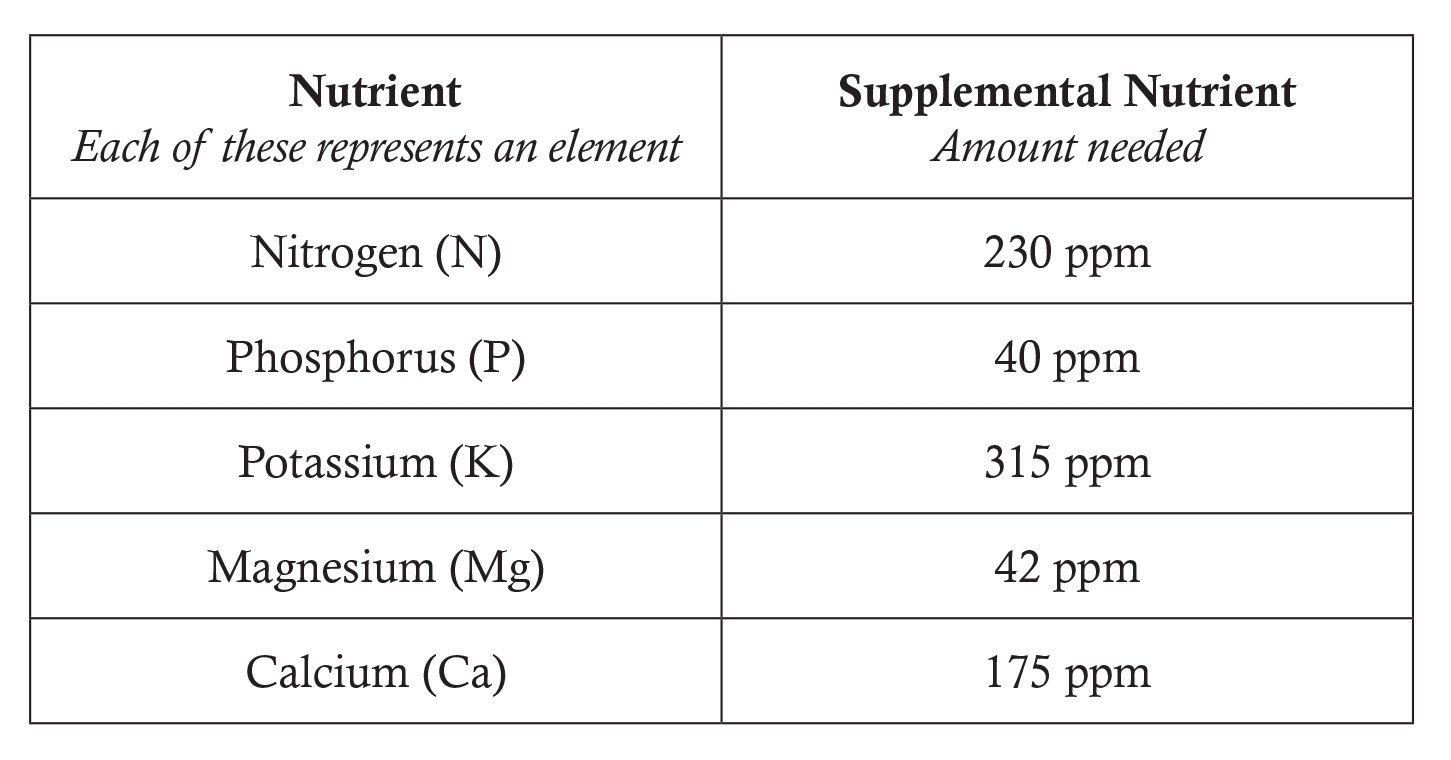
- Students may choose to apply one of the following fertilizer compounds: Use a document projector to display this chart to the class. Have students use a periodic table of the elements from their textbooks or project one on the board. Explain to students that each subscript represents the number of atoms of each element in the fertilizer compound.
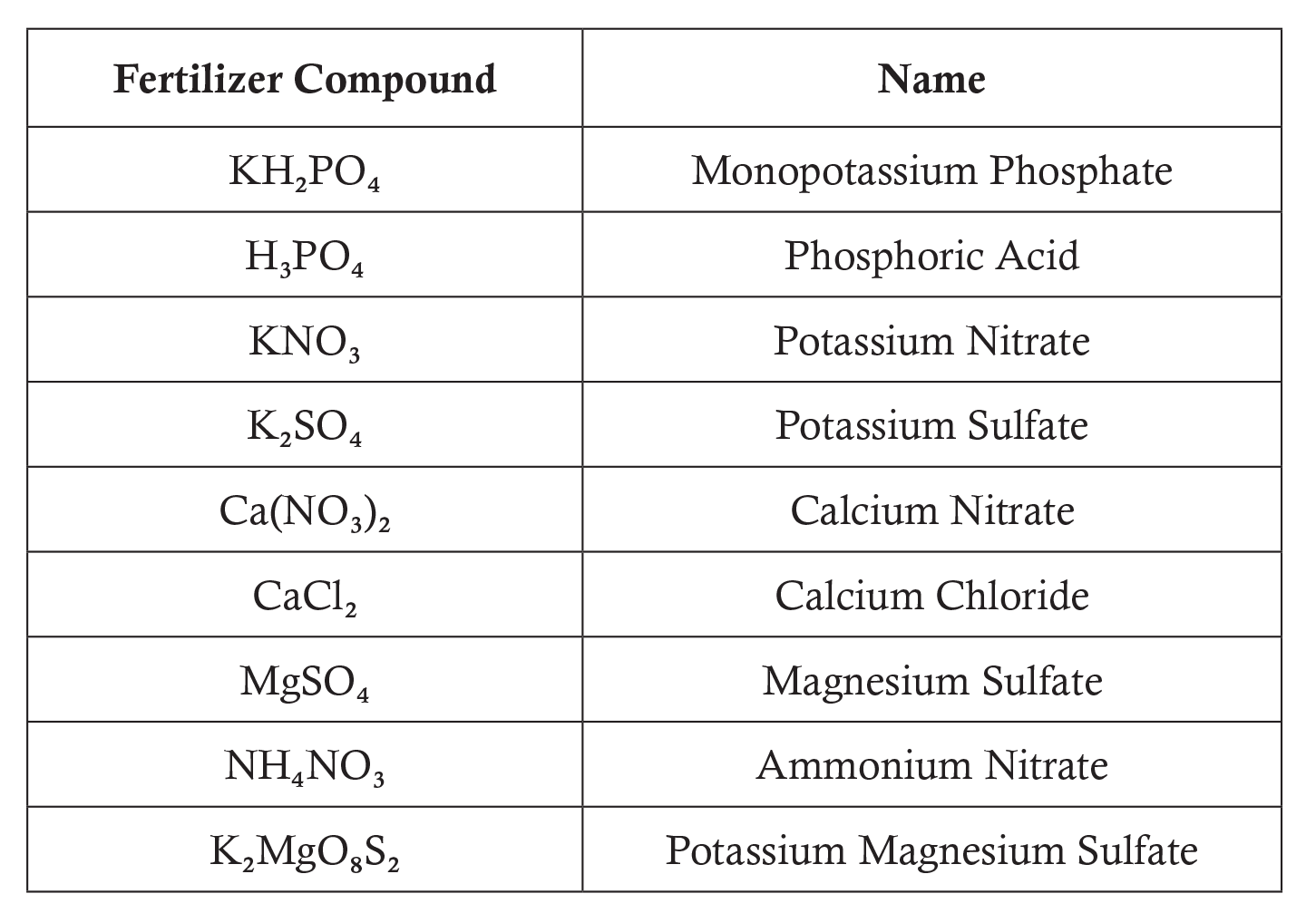
- Do one example with your class. After students complete all of the problems, review the solutions as a class. Discuss how you might apply this lesson to an actual school farm or community garden.
Variations
- Rather than assigning a nutrient deficiency and the supplemental amount needed, distribute photos of plants with nutrient deficiencies. Assign students homework that includes identifying the nutrient deficiency and the recommended application in ppm. The Internet has a wide range of resources. Recommend that students use university websites as a primary source.
ELL Adaptations
- Ask students to write a few sentences about the importance of precise measurements when preparing fertilizer then have them speak about the process with another student.
- Use an overhead projector to demonstrate complex math problems.
Elaborate
-
Have students research plant nutrients to determine if there are any elements that can be directly applied to plants as fertilizer. Investigate the chemical properties of these elements to determine why they are suitable for individual application.
Evaluate
After conducting these activities, review and summarize the following key concepts:
- Farmers produce our food.
- Plant-based food is typically grown in the soil. Plants use the available soil nutrients.
- Fertilizer can be used to replenish soil nutrients and allow for future plant growth and food production.
- Managing soil nutrients gets more and more important as our population grows to attain sustainability.
Acknowledgements
This lesson was funded in 2011 by the California Department of Food and Agriculture’s (CDFA) Fertilizer Research and Education Program (FREP). Chemistry, Fertilizer, and the Environment was designed to reinforce chemistry and environmental science concepts while educating students about the relationships between food, plant nutrients, farmers and the environment.
Executive Director: Judy Culbertson
Illustrator: Erik Davison
Layout and Design: Nina Danner
Recommended Companion Resources
Author
Organization
| We welcome your feedback! If you have a question about this lesson or would like to report a broken link, please send us an email at matrixelearning@gmail.com. If you have used this lesson and are willing to share your experience, we will provide you with a coupon code for 10% off your next purchase at AgClassroomStore. |
